Electron – What is it and Who Named it?
Let’s start from the very beginning — the universe’s tiniest fundamental units: particles.
- Everything in the universe that we can see or touch is made of matter. Matter is anything that has mass and takes up space (volume). Whether you’re looking at stars, planets, water, or gas — no matter what — it is made of matter.
- This matter is made of particles. It is understood that the fundamental particles making up everything in the universe are the same ones that comprise the familiar stuff of our everyday lives here on planet Earth.
- The three most important particles are protons, neutrons, and electrons — the backbone particles that make up most of the ordinary matter in the universe. These three are the subatomic particles of every atom, meaning every atom is made of electrons, protons, and neutrons.
(Remember: Atoms are the smallest building blocks of matter.)
What is an Electron?
An electron is a particle of electricity. It is a type of subatomic particle that orbits the atomic nucleus. Electrons are negatively charged and are considered fundamental particles, meaning they are not made of smaller parts. They are also stable — they live forever.
What do Electrons do?
Electrons play a major role in the atomic world. They:
- Carry electric charge
- Participate in electromagnetic interactions
- Contribute to the structure of atoms and matter
They also balance an atom’s charge. Atoms are made of positively charged protons, neutral neutrons, and negatively charged electrons.
In a neutral atom, the number of electrons is equal to the number of protons.
For example:
- Sodium (Na) has 11 protons and 11 electrons
- Potassium (K) has 19 protons and 19 electrons
- Carbon (C) has 6 protons and 6 electrons
In a neutral atom, there will always be the same number of protons as electrons.
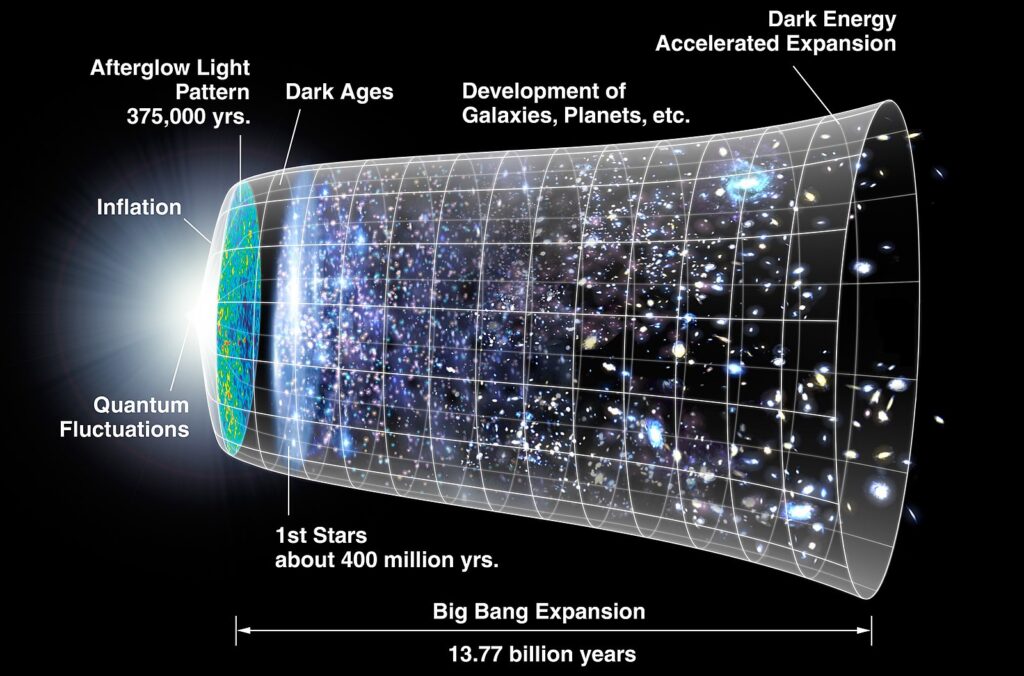
How are Electrons Created?
Electrons were created shortly after the Big Bang, in the early universe. They emerged naturally from energy during a time when the universe was so hot that photons (particles of light) could interact and form pairs of electrons and positrons.
- The idea that electrons formed after the Big Bang was explored in the 1940s by George Gamow, along with Ralph Alpher and Robert Herman, who developed the theory of nucleosynthesis — how elements formed after the Big Bang. Gamow applied his model to the question of how chemical elements were created.
That said, electrons can also be created today under certain physical conditions, such as:
- Particle accelerators (e.g., CERN)
- Beta decay (a type of radioactive decay)

Where are Electrons Found?
- As mentioned before, electrons are found in every atom, orbiting the nucleus. Since everything is made of atoms — planets, stars, and even us — electrons are everywhere.
- Electrons also exist as free particles. For example, in the Sun, atoms lose electrons, and these free electrons move independently in a state known as plasma.
- Even our tablets and phones rely on moving electrons. When we plug in our chargers, electrons flow through the charging cable — this flow is what we call electricity.
Irish Science Fact – Did You Know?
- It was George Johnstone Stoney, an Irish physicist, who, in 1891 introduced the the term “electron” as the fundamental unit quantity of electricity.
I have read a little bit about George Johnstone Stoney and turns out he had a very interesting life. Not only has he made contributions to cosmic physics and the theory of gases, but after attending Trinity College Dublin, he also worked as an astronomy assistant to William Parsons.
It is during this time of his life when Parsons had built the world’s largest telescope, the 72 inch telescope called the Leviathan of Parsonstown. And Stoney was part of this amazing experience.
- And yes, you can still see a reconstruction of the Leviathan of Parsonstown at Birr Castle Demesne in County Offaly, Ireland, though the original mirror is preserved in a museum.
- The telescope is located on the grounds of the castle and is a centrepiece of the Historic Science Centre, so be sure to check it out (it is around 1hour 20 minutes ways from Galway City).

References/Sources
The following books helped me with my research and are highly recommended:
- Why Does E=mc²? – Brian Cox & Jeff Forshaw
- Encyclopedia of Space – Miles Kelly
Please note: I carefully research each topic before publishing; however, some facts may change over time, so I appreciate your understanding in double-checking. All articles are thoroughly researched and inspired by published books. A list of sources is included in every article.
Please be kind, and have a nice day!
Nyx Log, Stardate 25091.6



Refer friends and colleagues—get paid for every signup!